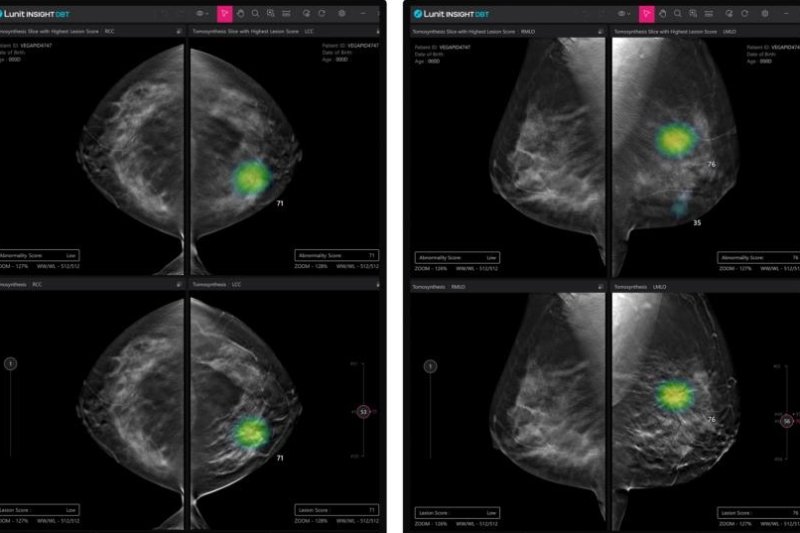
Lunit Insight DBT is designed to screen abnormal radiologic findings to detect breast cancers. Image courtesy of Lunit
SEOUL, March 21 (UPI) -- South Korean startup Lunit said its artificial intelligence-powered cancer-detecting solution has received approval for use from the European Union.
The Seoul-based Lunit, established in 2013, said Monday the new product launch would happen later this month in Europe as the result of receiving the CE mark from the EU medical devices regulatory body.
Lunit Insight Digital Breast Tomosynthesis is an AI-enabled software designed to detect breast cancers by identifying suspicious lesions via three-dimensional image analysis.
According to Lunit, the 3D solution exceeds the conventional two-dimensional technologies currently available in detecting tumors.
At its presentation during the 2023 European Congress of Radiology in Vienna this month, Lunit said its tomosynthesis diagnosis was nearly as accurate as that of physicians with 10-plus years experience in the field.
The research presented involved 162 South Korean women ages 52 or older who were analyzed using the new method during their visits to Korean hospitals.
Lunit said the product could become even more accurate via machine learning from the information that various medical institutes at home and abroad make public.
"We got the export license from South Korea's Ministry of Food and Drug Safety last month. And the CE mark now entitles us to ship the products to Europe," Lunit CEO Brandon Suh said in a statement.
"We are also targeting other countries like Australia, which honor the CE mark," he said.
Lunit is working to get the green light from other major countries, including the United States. It hopes to receive permission from the U.S. Food and Drug Administration by the end of September.
Having met the stringent requirements of the recently strengthened CE standard, the company expects approval from regulators in other regions to be forthcoming.
The share price of Lunit, which went public last year, shot up 5.23% Monday to dip back 3.5% Tuesday on the South Korean stock exchange.